NOX And Other Considerations
The fired heater industry has concentrated on the two primary sources of Nitrogen Oxides, NOx. These are normally referred to as Thermal NOx and Fuel NOx. The technology for NOx reduction via the combustion focuses on one or more of the strategies:
Both process modifications and new burner / furnace designs rely on the following concepts to implement the three main strategies for reducing NOx emissions:
| 1. Reduce peak temperatures |
| 2. Reduce the gas residence time in the hottest part of the flame zone |
| 3. Reduce the O2 content in the primary flame zone |
Burning with low excess air:
For many years, it was not uncommon to see furnaces operating with 50 to 100% excess air. It was simply easier for the operator to just make sure there was plenty of air. Of course this method of operation also resulted in reduced efficiency and more NOx generation. As fuel costs and environmental concerns has risen, these practices have changed. With the excess air at levels of 15 to 30%(lower for gas and higher for oil), the furnace could be operated with a minimum of monitoring. In recent years, development of advanced instrumentation has allowed continuous automatic furnace monitoring and control of excess air, and the percent excess air can now be reduced below the 10-30% limit. EPA tests concluded that a 19% average reduction of NOx can be achieved by reducing the percent excess air from an average of 20% to an average of 14%. This method is somewhat limited since it can cause other problems such as carbon monoxide and soot emissions.
Thermal NOx is the reaction of N2 with oxygen in the flame front of a burner. High flame temperatures contribute to thermal NOx formation. The original low NOx burners were designed with staged air (as opposed to the current technology using staged fuel). Staging either the air or the fuel accomplishes the same end result: a lowering of the flame temperature to reduce the formation of thermal NOx.
Staged combustion, or off stoichiometric combustion, burns the fuel in two or more steps. The initial or primary flame zone is fuel rich, and the secondary and following zones are fuel lean. Careful monitoring of the flue gas is necessary with this method to protect against CO formation and furnace smoking.
Air Staging :Burner designs for accomplishing vary among the burner manufacturers, but they basically work the same. Only a portion of the air flows across the fuel injection zone and this forms a fuel rich primary combustion zone where the fuel is only partially burned. As a result, only a portion of the fuel nitrogen decomposes to form molecular nitrogen, thus reducing NOx formation. And since, excess air is not available, Thermal NOx is also reduced. The remainder of the air is injected downstream to complete the combustion. NOx formation in this secondary zone is reduced since the products of combustion from the primary zone reduce the flame temperature and oxygen concentrations.
A staged air, combination oil and gas burner is shown below:
When firing gas fuels, thermal NOx can be controlled using fuel staging. The fuel is divided into two of more streams. Only a portion of the fuel is injected into the primary combustion zone. The NOx levels are very low because the flame temperature is low. The rest of the fuel requirement is introduced downstream. The NOx formation is lower in this secondary zone because the inerts from the primary zone depress the peak temperature of the secondary zone and reduce oxygen concentrations. Flame lengths in staged fuel burners are generally shorter and more defined compared to staged air burners. A lower excess air is achievable with this type burner.
A staged fuel gas burner is shown below: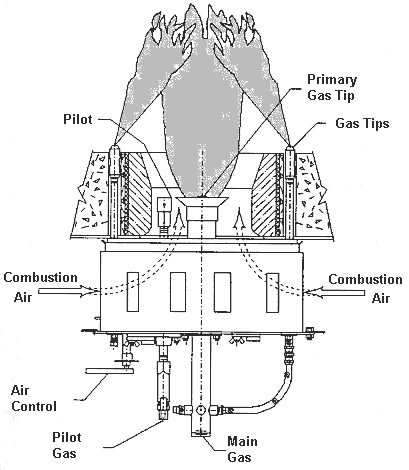
Acceptance of low NOx burners in the heater industry has been good. In fact, low NOx burners are now a standard part of new designs. Burner spacing can also affect NOx formation. In a large heater, there may be many burners with all the flames merging because of tight spacing, higher temperatures (hence, greater NOx formation) result, especially in the central portion of the burner matrix. Furnaces themselves can be designed in larger sizes. Larger furnaces inhibit NOx formation in similar ways as increased burner spacing. Larger enclosures provide more time for complete combustion using off stoichiometric combustion burners. Also, larger enclosures provide more tubes to achieve the same amount of heat transfer from lower temperature flames. Finally, larger enclosures, reduce turbulent mixing of the fuel and air, which also inhibits NOx formation (particularly fuel NOx).
Fuel NOxFuel NOx is NOx formed by nitrogen atoms that are chemically bound in the fuel. Liquid fuels almost always contain some nitrogen, and as a rule, liquid fuels make more NOx than burning gases. Generally single nitrogen atoms are the most reactive. Amines and ammonia in the fuel contribute greatly to fuel NOx formation.
Flue gas recirculation:Flue gas recirculation is simply the rerouting of some of the flue gas back to the furnace. The quenching effect from the CO2 rich flue gas lowers the O2 concentration in the combustion zone, as well as the adiabatic flame temperature. Up to 20% flue gas recirculation is very effective. In addition to requiring large ducts, fans, and dampers, additional controls will be required. The additional gas flow through the firebox and flues must be taken into account in the furnace design.
Steam Injection:Water injection (or steam injection) can be an effective means of reducing flame temperatures, thus reducing thermal NOx. This method injects steam into the flame zone to reduce flame temperatures. Steam injection into the fuel gas at rates up to 30% by weight have been shown to be quite effective.
Above, we have discussed the ways to modify the combustion process to prevent the formation of NOx. But, post flame NOx control may also be used. The more common post flame treatment is use of Selective Catalytic Reduction (SCR) to convert the NOx to N2.
Selective Catalytic ReductionPostflame treatment processes add a reducing agent to the combustion gas stream to take oxygen away from NO. For large heaters ammonia is used as the reducing agent. The desired reaction is
This requires 2/3 ammonia molecules for each molecule of NO. However, there is always some oxygen present, which leads to reactions like the following:
In this reaction one ammonia molecule is required for each molecule of NO. NO2 is reduced by
These reactions can be carried out over a zeolite catalyst at 700 o to 800 °F. This catalytic process has been employed on a large scale and gives up to 85% reduction in NO2.
Titanium and Vanadium CatalystsThe first successful commercial NO2 SCR system was built by Hitachi Zosen in 1975. Since then, the technology has become well established world wide. The catalyst used is a honeycomb shaped metal or ceramic plate with active sites made up of a mixture of titanium and vanadium oxides. The catalysts are selective to optimize NO2 activity and minimize SO2 oxidation. The honeycomb shape is desirable to minimize plugging if particulates are present. The best temperature range for SCR catalyst activity and selectivity is from 700 ° to 800 °F. Ammonia is injected downstream from the combustion zone and upstream of the catalyst.
In process heaters the catalyst undergoes slow deactivation over a long period of time. The thickness and hence the space velocity of the honeycomb blocks is typically specified for a three year life. As the activity of the catalyst decreases, the amount of excess ammonia required for the desired conversion increases. Maximum allowable ammonia "slip" is specified when the catalyst is purchased. Ammonia slip of 10 ppm is typical. The catalyst is manufactured in blocks that are held in place in the stack gas ductwork by a stainless steel T-bar grid.
The ammonia is injected upstream of the catalyst grid through its own injection grid in a turbulent zone. Anhydrous or aqueous ammonia can be used, depending on the policies of the plant where the system is to be installed. In the case of aqueous ammonia, the material can either be vaporized first and injected as a vapor, or in some duct configurations, it can sprayed in through an atomizing spray nozzle.
SCR catalyst substrate is recyclable and can be regenerated by the manufacturer if it has not been deformed or damaged by heat. Most often a spare charge is kept on hand for changeout during routine turnarounds and the spent material is sent in for regeneration. There is about a 30% cost savings in reusing the substrate over a new purchase and it eliminates catalyst disposal cost concerns.
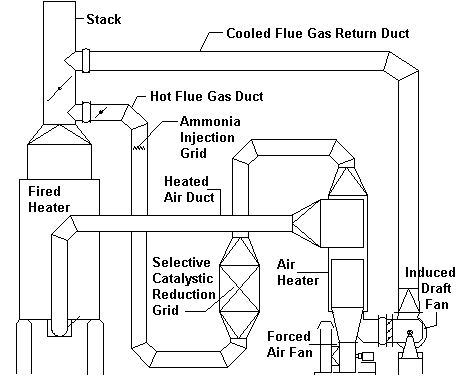
Below is a sketch of a direct fired heater system utilizing an SCR unit and Air Preheat system.
Selective non-catalytic reduction involves the injection of a NOx reducing agent, such as ammonia or urea, into the heater flue gases at a temperature of approximately 1400-1600 °F. The ammonia or urea breaks down the NOx in the flue gases into water and atmospheric nitrogen. Selective non-catalytic reduction reduces NOx up to 70%. Because of the high temperature injection point required for this method, it is not widely used in the heater industry.
Carbon Monoxide (CO) Emissions High flame temperatures and intimate air/fuel mixing are essential for low CO emissions. Some NOx control technologies used to reduce NOx levels by lowering flame temperatures by modifying air/fuel mixing patterns can result in higher CO levels. Sulfur Compounds (SOx)The primary reason sulfur compounds, or SOx, are classified as a pollutant is because they react with water vapor (in the flue gas and atmosphere) to form sulfuric acid mist. Airborne sulfuric acid has been found in fog, smog, acid rain, and snow. Sulfuric acid has also been found in lakes, rivers, and soil. The acid is extremely corrosive and harmful to the environment.
The combustion of fuels containing sulfur results in pollutants occurring in the forms of SO2 (sulfur dioxide) and SO3 (sulfur trioxide), together referred to as SOx (sulfur oxides). The level of SOx emitted depends directly on the sulfur content of the fuel. The level of SOx emissions is not dependent on boiler size or burner design. Typically, about 95% of the sulfur in the fuel will be emitted as SO2, 1-5% as SO3, and 1-3% as sulfate particulate. Sulfate particulate is not considered part of the total SOx emissions.
Historically, SOx pollution has been controlled by either dispersion or reduction. Dispersion involves the utilization of a tall stack, which enables the release of pollutants high above the ground and over any surrounding buildings, mountains, or hills, in order to limit ground level SOx emissions. Today, dispersion alone is not enough to meet more stringent SOx emission requirements; reduction methods must also be employed.
Methods of SOx reduction in fired heaters consits of switching to low sulfur fuel. Heater design primarily requires taking the sulfur into account in the convection section. See section 5, page 6 for more discussion on the acid dew point of flue gases.
Carbon Monoxide (CO)Carbon monoxide is a pollutant that is readily absorbed in the body and can impair the oxygen-carrying capacity of the hemoglobin. Impairment of the body's hemoglobin results in less oxygen to the brain, heart, and tissues. Even short-term over exposure to carbon monoxide can be critical, or fatal, to people with heart and lung diseases. It may also cause headaches and dizziness in healthy people.
During combustion, carbon in the fuel oxidizes through a series of reactions to form carbon dioxide (CO2). However, 100 percent conversion of carbon to CO2 is rarely achieved in practice and some carbon only oxidizes to the intermediate step, carbon monoxide.
Older heaters generally have higher levels of CO than new equipment because CO has only recently become a concern and older burners were not designed to achieve low CO levels. In today's equipment, high levels of carbon monoxide emissions primarily result from incomplete combustion due to poor burner design or firing conditions (for example, an improper air-to-fuel ratio) or possibly a leaky furnace. Through proper burner maintenance, inspections, operation, or by upgrading equipment or utilizing an oxygen control package, the formation of carbon monoxide can be controlled at an acceptable level.
Particulate Matter (PM)Emissions of particulate matter (PM) from combustion sources consist of many different types of compounds, including nitrates, sulfates, carbons, oxides, and any uncombusted elements in the fuel. Particulate pollutants can be corrosive, toxic to plants and animals, and harmful to humans.
Particulate matter emissions generally are classified into two categories, PM and PM10. PM10 is a particulate matter with a diameter less than 10 microns. All particulate matter can pose a health problem. However, the greatest concern is with PM10, because of its ability to bypass the body's natural filtering system.
PM emissions are primarily dependent on the grade of fuel fired in the boiler. Generally, PM levels from natural gas are significantly lower than those of oils. Distillate oils result in much lower particulate emissions than residual oils.
When burning heavy oils, particulate levels mainly depend on four fuel constituents: sulfur, ash, carbon residue, and asphalenes. These constituents exist in fuel oils, particularly residual oils, and have a major effect on particulate emissions. By knowing the fuel constituent levels, the particulate emissions for the oil can be estimated.
For most heater applications, the most effective method is to utilize clean fuels. The emission levels of particulate matter can be lowered by switching from a residual to a distillate oil or by switching from a distillate oil to a natural gas. Additionally, through proper burner set-up, adjustment and maintenance, particulate emissions can be minimized, but not to the extent accomplished by switching fuels.
Volatile Organic Compounds (VOCs)/HYDROCARBONS (HC)Volatile organic compounds, or VOCs, are compounds containing combinations of carbon, hydrogen, and sometimes oxygen. VOCs vaporize easily once emitted into the air and are of concern because of their role in ground level ozone formation. In reference to heater performance, they are often referred to as hydrocarbons and generally are divided into two categories Ñ methane and non-methane. Formation of VOCs in fired heaters primarily result from poor or incomplete combustion due to improper burner set-up and adjustment. To control VOC emissions from fired heaters, no auxiliary equipment is needed; properly maintaining the burner/control package will keep VOC emissions at a minimum. Proper maintenance includes keeping the air/fuel ratio at the manufacturer's specified setting, having the proper air and fuel pressures at the burner, and maintaining the atomizing air pressure on oil burners at the correct levels. An improperly maintained control/burner package can result in VOC levels over 100 times the normal levels.
Correcting Emissions To 3% Oxygen (15% Excess Air)The following equation shows how to correct emission readings to 3% oxygen (15% excess air). Because heaters don't always operate at 3% oxygen, it is necessary to convert ppm values measured at various excess air levels to 3% oxygen for comparison and regulation compliance purposes. To correct emission levels to 3% oxygen that are referenced to excess air levels other than 3%, use the following equation.
Example: What is the NOx level corrected to 3% oxygen for a measured level of 36 ppm at 8.3% oxygen?
Disclaimer:
The formulas and correlations presented herein are all in the public domain and are to be used only as a learning tool. Note that any product, process, or technology in this document may be the subject of other intellectual property rights reserved by sponsors or contributors to this site. This publication is provided as is, without any warranty of any kind, either expressed or implied, including, but not limited to, the implied warranties of fitness for a particular purpose, or non-infringement.
The formulas, correlations, and methods presented herein should not be considered as being recommended by or used by the sponsors of this site. The purpose of this site is educational and the methods may or may not be suitable for actual design of equipment. Only a fired heater design engineer is qualified to decide if a calculation or procedure is correct for an application.